Quarantine can end after Day 10 without testing and if no symptoms have been reported during daily monitoring.
Local public health authorities determine and establish the quarantine options for their jurisdictions. CDC currently recommends a quarantine period of 14 days. However, based on local circumstances and resources, the following options to shorten quarantine are acceptable alternatives.
- Quarantine can end after Day 10 without testing and if no symptoms have been reported during daily monitoring.
- With this strategy, residual post-quarantine transmission risk is estimated to be about 1% with an upper limit of about 10%.
- When diagnostic testing resources are sufficient and available (see bullet 3, below), then quarantine can end after Day 7 if a diagnostic specimen tests negative and if no symptoms were reported during daily monitoring. The specimen may be collected and tested within 48 hours before the time of planned quarantine discontinuation (e.g., in anticipation of testing delays), but quarantine cannot be discontinued earlier than after Day 7.
- With this strategy, the residual post-quarantine transmission risk is estimated to be about 5% with an upper limit of about 12%.
In both cases, additional criteria (e.g., continued symptom monitoring and masking through Day 14) must be met and are outlined in the full text.
Background
Quarantine is used to separate someone who might have been exposed to COVID-19 and may develop illness away from other people. Quarantine helps prevent spread of disease that can occur before a person knows they have the virus. CDC recognizes that any quarantine shorter than 14 days balances reduced burden against a small possibility of increasing the spread of the virus.
The recommendation for a 14-day quarantine was based on estimates of the upper bounds of the COVID-19 incubation period. Quarantine’s importance grew after it was evident that persons are able to transmit SARS-CoV-2 before symptoms develop, and that a substantial portion of infected persons (likely somewhere between 20% to 40%1) never develop symptomatic illness but can still transmit the virus. In this context, quarantine is a critical measure to control transmission.
Quarantine is intended to reduce the risk that infected persons might unknowingly transmit infection to others. It also ensures that persons who become symptomatic or are otherwise diagnosed during quarantine can be rapidly brought to care and evaluated. However, a 14-day quarantine can impose personal burdens that may affect physical and mental health as well as cause economic hardship that may reduce compliance. Implementing quarantines can also pose additional burdens on public health systems and communities, especially during periods when new infections, and consequently the number of contacts needing to quarantine, are rapidly rising. Lastly, the prospect of quarantine may dissuade recently diagnosed persons from naming contacts and may dissuade contacts from responding to contact tracer outreach if they perceive the length of quarantine as onerous.
Reducing the length of quarantine will reduce the burden and may increase community compliance. This document lays out evidence to support two options to shorten the quarantine period. Shortening quarantine may increase willingness to adhere to public health recommendations but will require evaluation; not only in terms of compliance with quarantine and contact tracing activities, but also for any potential negative impacts such as post-quarantine transmission. Any option to shorten quarantine risks being less effective than the currently recommended 14-day quarantine. The variability of SARS-CoV-2 transmission observed to-date indicates that while a shorter quarantine substantially reduces secondary transmission risk, there may be settings (e.g., with high contact rates) where even a small risk of post-quarantine transmission could still result in substantial secondary clusters.
Testing during quarantine to reduce the burden; modeled outcomes
CDC scientists modeled the residual post-quarantine transmission risk, expressed as a percent of total transmission, per day of quarantine if quarantine were discontinued that day. The model2 estimated the effect of having a negative diagnostic test prior to discontinuation of quarantine as well as options without any testing, in all cases combined with daily symptom monitoring for COVID-19 illness both during quarantine and after its discontinuation through Day 14. Day 0 was defined as the day of infection*. The precise time of infection is rarely known, but in practice quarantine timing would be based on the last known or possible exposure to a person with SARS-CoV-2 infection. For testing, the model assumed that the diagnostic specimen would be collected up to 48 hours prior to the proposed end of quarantine. For example, for a modeled quarantine that would end on Day 7, the diagnostic specimen could be collected starting on Day 5 or thereafter. These estimates assume that when diagnostic testing was performed, results were available after the diagnostic specimen’s collection and before the end of quarantine. The presence of any symptoms would lead to diagnostic testing and management as infected if the test result were positive.
Results are shown in the Figure and Table. Although daily monitoring for symptoms of COVID-19 illness reduced the estimated post-quarantine transmission risk, addition of diagnostic testing for a person who remained asymptomatic substantially reduced the estimated post-quarantine transmission risk, especially after Day 5. For instance, at Day 10 with symptom monitoring but without diagnostic testing, the estimated residual post-quarantine transmission risk was 1.4% (range 0.1%-10.6%). However, with the addition of diagnostic testing of a specimen collected up to 48 hours before Day 10, the estimated post-quarantine transmission risk was reduced to 0.3% (range 0.0%-2.4%) for RT-PCR testing, and 1.1% (0.1%-9.5%) for antigen testing with a test that had a diagnostic sensitivity of 70%.
* The model estimates what occurs in a person who was infected in terms of how likely they would be to infect others were quarantine discontinued.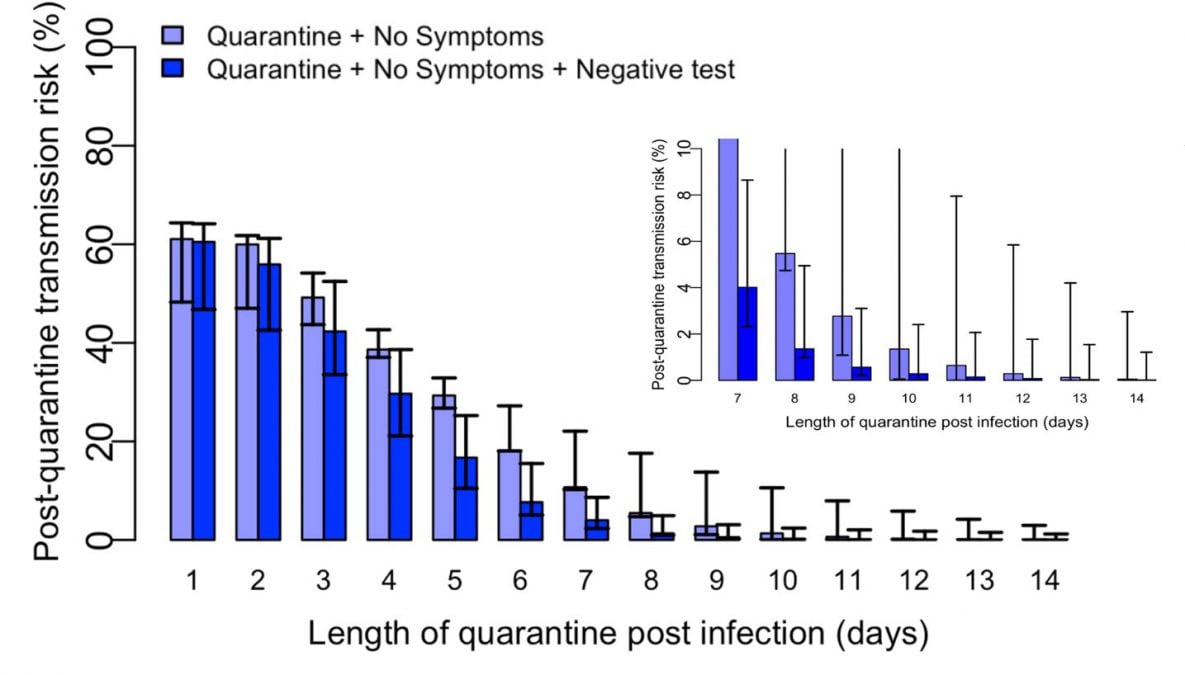
Figure. Modeled estimates of post-quarantine transmission risk quarantine duration. The light blue bars indicate the daily post-quarantine transmission risk if there is no clinical evidence of COVID-19 elicited during daily symptom monitoring. The dark blue bars indicate the post-quarantine transmission risk with the addition of a negative RT-PCR result from a specimen collected 24-48 hours prior.
Table. Estimated residual post-quarantine transmission risk with and without a negative diagnostic test of a specimen collected within 48 hours prior to discontinuation of quarantine on the indicated day for a person monitored daily for symptoms and who has remained asymptomatic until quarantine is discontinued as well as through Day 14. Published data were applied to model residual post-quarantine transmission risk using RT-PCR3,4; for antigen testing, a diagnostic sensitivity of 70% was applied.
| Planned day after which quarantine is completed and can be discontinued | Residual post-quarantine transmission risk (%) with and without diagnostic testing of a specimen within 48 hours before time of planned discontinuation of quarantine | |||||
| No testing | RT-PCR testing | Antigen testing | ||||
| Median | Range | Median | Range | Median | Range | |
| 7 | 10.7 | 10.3-22.1 | 4.0 | 2.3-8.6 | 5.5 | 3.1-11.9 |
| 10 | 1.4 | 0.1-10.6 | 0.3 | 0.0-2.4 | 1.1 | 0.1-9.5 |
| 14 | 0.1 | 0.0-3.0 | 0.0 | 0.0-1.2 | 0.1 | 0.0-2.9 |
Additional modeling by groups outside of CDC have produced similar findings that align with those presented above.
- Quilty and Clifford et al.5 (preprint pending peer review) modeled the median transmission potential averted by various quarantine strategies with and without testing. They estimated that 14 days of quarantine without testing was approximately equivalent to 7 days of quarantine when a specimen collected on the last quarantine day tests negative by RT-PCR.
- Wells et al.6 (preprint pending peer review) estimated the post-quarantine transmission risk (PQTR) for persons who have remained asymptomatic during quarantine based on RT-PCR testing performed within 24 hours prior to the date quarantine was discontinued. With average incubation periods of 5.2 days and 8.3 days, the PQTR fell below 1% after a 5-day or 7-day quarantine, respectively.
- Higher prevalence translates to greater pre-test probability that an exposed person has been infected. Modelers from the University of Utah School of Medicine estimated the post-quarantine transmission risk accordingly (unpublished data). At community prevalences of 1%, 3% and 5%, the post-quarantine transmission risk at Day 7 of quarantine were 0.25%, 0.84%, and 1.38%, respectively, with a diagnostic test that had 90% sensitivity.7
Adding testing at entry to quarantine
Adding testing at entry to quarantine provided little additional benefit in terms of reduction in post-quarantine transmission risk.6 However, testing may be useful to identify infected persons without symptoms for contact tracing efforts, if sufficient resources allow.
- CDC recommends the following alternative options to a 14-day quarantine:
- Quarantine can end after Day 10 without testing and if no symptoms have been reported during daily monitoring.
- With this strategy, residual post-quarantine transmission risk is estimated to be about 1% with an upper limit of about 10%.
- When diagnostic testing resources are sufficient and available (see bullet 3, below), then quarantine can end after Day 7 if a diagnostic specimen tests negative and if no symptoms were reported during daily monitoring. The specimen may be collected and tested within 48 hours before the time of planned quarantine discontinuation (e.g., in anticipation of testing delays), but quarantine cannot be discontinued earlier than after Day 7.
- With this strategy, the residual post-quarantine transmission risk is estimated to be about 5% with an upper limit of about 12%.
- Quarantine can end after Day 10 without testing and if no symptoms have been reported during daily monitoring.
- Persons can discontinue quarantine at these time points only if the following criteria are also met:
- No clinical evidence of COVID-19 has been elicited by daily symptom monitoring† during the entirety of quarantine up to the time at which quarantine is discontinued; and,
- Daily symptom monitoring continues through quarantine Day 14; and,
- Persons are counseled regarding the need to adhere strictly through quarantine Day 14 to all recommended non-pharmaceutical interventions (NPIs±, a.k.a. mitigation strategies), especially. They should be advised that if any symptoms develop, they should immediately self-isolate and contact the local public health authority or their healthcare provider to report this change in clinical status.
- Testing for the purpose of earlier discontinuation of quarantine should be considered only if it will have no impact on community diagnostic testing. Testing of persons seeking evaluation for infection must be prioritized.
- Persons can continue to be quarantined for 14 days without testing per existing recommendations. This option maximally reduces risk of post-quarantine transmission risk and is the strategy with the greatest collective experience at present.
These recommendations for quarantine options shorter than 14 days balance reduced burden against a small but non-zero risk of post-quarantine infection that is informed by new and emerging science.
† Monitoring can be conducted using any method acceptable to local public health authorities and could include self-monitoring using an approved checklist of signs and symptoms, direct contact daily by public health authorities or their designates, or automated communications systems (e.g., on-line or texting self-checkers).
± NPIs that can be practiced by individuals include the following: correct and consistent mask use, social distancing, hand and cough hygiene, environmental cleaning and disinfection, avoiding crowds, ensuring adequate indoor ventilation, and self-monitoring for symptoms of COVID-19 illness. These are also summarized here.
Persons who must quarantine together, such as households
Quarantine is intended to physically separate a person exposed to COVID-19 from others. Secondary transmission of infection is especially efficient within households.8-10 Thus, when housing is shared (e.g., households or co-housed persons such as families, incarcerated persons, students, or military recruits), every effort should be made to physically separate the quarantined person from others such as by having the quarantined person reside alone in a separate closed room or closed area and with exclusive use of their own bathroom. When this separation is not possible, then the household members risk exposure to COVID-19 if the quarantined person develops the illness. People who are quarantined with others, as well as the person in quarantine, should take steps to prevent spread of infection within the household (e.g., NPIs, a.k.a. mitigating strategies). If the quarantined person is diagnosed with COVID-19, co-housed persons will require evaluation as contacts.
Additional considerations
- Burden of additional testing: Diagnostic testing during quarantine will require capacity to produce results within a short period of time, and to report these additional results to public health authorities in a timely manner.
- Equity: Public health authorities that choose to use diagnostic testing during quarantine should strive to ensure equitable access for all affected persons and communities within their jurisdictions.
- Serologic testing: The utility of serologic testing to provide evidence of prior infection that would permit exclusion from quarantine has not been established and is not recommended for this purpose at this time
- Monitoring and evaluation of changes to quarantine recommendations: Documented data-driven experience is critical to ensure that these options for quarantine achieve an acceptable balance of risk to benefit once operationalized. CDC strongly encourages collection of data related to the effect of the recommended changes made herein to include (but not limited to): compliance with contact tracing (e.g., engaging with public health to identify contacts), willingness and ability to complete quarantine, change in burden to public health, and observed post-quarantine transmission rates.
These recommendations are based on the best information available in November 2020 and reflect the realities of an evolving pandemic. CDC will continue to closely monitor the evolving science for information that would warrant reconsideration of these recommendations.
References
- Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Annals of internal medicine. 2020.10.7326/m20-3012.
- Johansson MS, Wolford H, Paul P, et al. Reducing travel-related SARS-CoV-2 transmission with layered mitigation measures: symptom monitoring, quarantine, and testing medRxiv. 2020. https://doi.org/10.1101/2020.11.23.20237412external icon.
- Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Annals of internal medicine. 2020.10.7326/m20-1495.
- Clifford S, Quilty BJ, Russell TW, et al. Strategies to reduce the risk of SARS-CoV-2 reintroduction from international travellers. medRxiv. 2020.10.1101/2020.07.24.20161281; . https://doi.org/10.1101/2020.07.24.20161281external icon; .
- Quilty BJ, Clifford S, Flasche S, Kucharski AJ, CMMID COVID-19 Working Group, Edmunds WJ. Quarantine and testing strategies in contact tracing for SARS-CoV-2: a modelling study. medRxiv. 2020. https://doi.org/10.1101/2020.08.21.20177808external icon.
- Wells CR, Townsend JP, Pandey A, et al. Optimal COVID-19 quarantine and testing strategies. medRxiv. 2020.10.1101/2020.10.27.20211631. https://doi.org/10.1101/2020.10.27.20211631external icon;.
- Khader K, Thomas A, Hersh AL, Samore MH. Duration of quarantine and post-test risk of infection among individuals exposed to SARS-CoV-2. unpublished data shared through personal communication. 2020.
- Liu Y, Eggo RM, Kucharski AJ. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet. 2020;395(10227):e47.10.1016/S0140-6736(20)30462-1. https://www.ncbi.nlm.nih.gov/pubmed/32113505external icon.
- Ng OT, Marimuthu K, Koh V, et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis. 2020.10.1016/S1473-3099(20)30833-1. https://www.ncbi.nlm.nih.gov/pubmed/33152271external icon.
- Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 Infections in Households – Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634.10.15585/mmwr. mm6944e1. https://www.ncbi.nlm.nih.gov/pubmed/33151916external icon.
Last Updated Dec. 2, 2020Content source: National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases
Source: https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-options-to-reduce-quarantine.html
